Similarly, when a normal adult sits quietly in an awake but relaxed state or meditates, the brain shows a great increase in alpha (8-12 Hz) activity and some theta (4-7 Hz) activity but relatively little delta or beta activity. On the other hand, a person who is alert and mentally active will show increased amounts of higher frequency beta activity (13-30 Hz). At the higher end (>25 hz), beta waves are associated with hypervigilance and feelings of anxiety.
Due to stress, neurotransmitter imbalances, genetic factors, brain injury, or other trauma, people may produce too much or too little of certain brain waves for certain activities. For example, many people who have trouble falling asleep and/or experience frequent waking during the night, do not produce enough very low frequency delta (1-4 Hz) and theta (4-8 Hz) brain waves at bedtime and, when they do manage to fall asleep, will experience frequent bursts of higher frequency alpha (8-12 Hz) activity which will bring them up into wakefulness... a problem referred to as "alpha intrusion sleep disorder".
Another common problem is seen when the brain chronically produces too much high-frequency beta (20-30 Hz) activity and the person feels constantly anxious, “wired” and hypervigilant and is unable to relax.
A different problem is seen in persons with Attention-Deficit Disorder, these people frequently have brains that produce too much slow wave theta (4-8 Hz) activity, especially in the frontal executive areas of the brain, when they try to do such mental tasks as reading or listening to a teacher lecture.
All of these problems can be helped with AVE. For the person who cannot sleep at night, AVE can be used to stimulate the brain at very low delta frequencies to enhance production of slow delta waves in the brain. For the person with anxiety and hypervigilance, AVE can be used to stimulate an increase in theta and alpha wave activity which will result in feelings of relaxation. Similarly, attention-deficit can be treated by stimulating an increase in brain activity within the lower beta range of 12-16 Hz, which is associated with relaxed attentiveness and mental focus.
Mood disorders such as depression and seasonal affective disorder may improve with AVE stimulation in the low and middle beta range (13-20 Hz).
What problems can be treated with AVE?
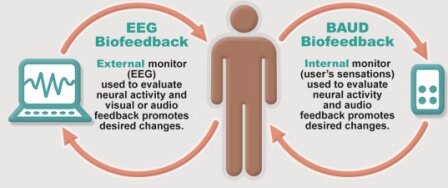
 AVE is believed to achieve its effects through several mechanisms simultaneously. These include: altered EEG activity, dissociation/hypnotic induction, limbic stabilization, improved neurotransmitter production, and altered cerebral blood flow/perfusion. AVE effects on the EEG are found primarily over the sensory-motor strip, both frontally and parietally in the somato-sensory regions and somewhat less within the prefrontal and frontal cortices. It is within these areas that motor activation, attention, executive function, and somato-sensory (body) awareness is primarily mediated.
AVE is believed to achieve its effects through several mechanisms simultaneously. These include: altered EEG activity, dissociation/hypnotic induction, limbic stabilization, improved neurotransmitter production, and altered cerebral blood flow/perfusion. AVE effects on the EEG are found primarily over the sensory-motor strip, both frontally and parietally in the somato-sensory regions and somewhat less within the prefrontal and frontal cortices. It is within these areas that motor activation, attention, executive function, and somato-sensory (body) awareness is primarily mediated.
There is a rapidly growing clinical and research literature supporting the use of AVE in the treatment of the following disorders…
· Anxiety
· Depression
· Seasonal Affective Disorder
· Attention Deficit Disorder
· Insomnia
· Posttraumatc Stress Disorder
AVE is especially effective for most people as a means of inducing deep relaxation and relieving stress. Successful entrainment most often results in a meditative, peaceful kind of dissociation, where the individual experiences a loss of somatic and cognitive awareness and an altered state of consciousness.
AVE can be a beneficial “stand-alone” or adjunctive treatment for many disorders and can produce changes in many conditions in only a few treatment sessions. AVE is also frequently used by therapists in conjunction with other EEG neurotherapies such as EEG neurofeedback and/or Cranial Electrotherapy Stimulation (CES), or with psychotherapy.
To view a YouTube video of David Siever, CEO of Mind Alive Inc., lecturing on Audio-Visual Entrainment at the March 2011 annual meeting of the Association for Psychophysiology and Biofeedback in New Orleans, GOTO:
What does AVE feel like?
AVE in the clinic usually involves having the client sit in a comfortable chair in a quiet room with eyes closed and wearing a set of special eyeglasses and a set of headphones. The eyeglasses have little LED lights built into them and these lights gently flash against the closed eye lid and are perceived as a diffuse pattern of flickering light. The headphones will emit a pulsating tone that will be synchronized with the flashing lights. The intensity of the lights and the volume of the tones are adjusted to be completely comfortable.
Treatment sessions usually last about 20-30 minutes.
With eyes closed, clients will experience incessantly changing patterns against their eyelids, that will change in perceived color as a function of the rate of flashing... between 10-15 Hz orange to red; above 15 Hz green and blue; above 18 Hz white and grey.
Dissociation begins after about 5-10 minutes from properly administered AVE. a restabilization effect occurs where muscles relax, electrodermal (sweat gland) activity decreases, peripheral blood flow stabilizes, breathing slows and becomes diaphragmatic and relaxed, and heart rate slows and becomes more uniform and smooth. Entrainment within the theta-alpha frequency (7-10 Hz) range has been shown to easily induce hypnosis and about 80% of people experiencing slow alpha entrainment will experience light to deep hypnotic trance within a few minutes.
Most clients are left feeling relaxed and alert after an AVE session— in what psychologists call an “alpha state”. Positive changes are usually felt within one to three sessions and include improvements in mental clarity, uplifted mood, increased mental alertness and energy, increased feelings of calm. Stable long-term effects may require regular daily AVE treatment sessions over periods of a month or longer. Clinical experience strongly suggests that with repeated training, AVE cultivates an adaptive self-regulation process and provides exogenous signals to entrain cortical activity to desirable freuencies and brain states.
What are the adverse effects of AVE?
While AVE has a demonstrated track record of safety, especially in comparison to many of the alternative pharmaceutical treatments for similar conditions, there is a small risk of seizure for persons who are epileptic or have a history of seizures or brain injury. The prevalence of photosensitive epilepsy is about 1 in 4000 children and young adults, lesser in older adults and slightly higher in females than males. Some people find the light stimulation, even when set very low, to be irritating. In some cases, headaches, vertigo or paradoxical feelings of anxiety may be triggered. Such adverse reactions are always temporary and resolve quickly once the stimulation is stopped. In some individuals who have a long history of chronic anxiety with associated high levels of sympathetic arousal, the quick reduction in sympathetic arousal and induction of physiological relaxation may cause a paradoxical feeling of anxiety and panic because the individual no longer recognizes the reduction in sympathetic arousal as healthy. Such individuals should not continue attempting AVE without seeking further professional supervision and help in addressing their anxiety and developing relaxation/meditation skills.
What is the evidence for the effectiveness of AVE?
Research studies of AVE go back as far as the 1950s but it is really only in the last three to four decades that AVE has developed into a clinical technique for treating brain-based problems. While there continues to be a serious lack of large sample, controlled research studies on AVE, there are literally hundreds of clinical reports in the professional literature showing AVE to hold significant promise in the treatment of anxiety and depression, seasonal affective disorder, insomnia, and attention-deficit disorder. There is also good support for the use of AVE as a stress management and relaxation tool as well as for pain relief. Clinically, AVE has been used quite successfully to induce deep relaxation and help in hypnosis. There is evidence that AVE can be used to “sharpen” cognitive functioning in the elderly who are showing early signs of age-related dementia.
AVE has been safely and effectively used by hundreds of thousands of individuals to quickly and effectively modify conditions of high autonomic activation and over- or under-aroused states of mind, bringing about a return to homeostasis.
Treatment of Depression: A 2009 study by David Cantor and Emily Stevens at the Psychological Sciences Institute in Duluth, Georgia examined the effects of 4 weeks of daily 14 Hz audio-visual EEG entrainment on 16 depressed subjects. They reported that 4 weeks of AVE treatment was associated with a significant reduction in depressive symptoms as well as EEG changes over time in cortical regions associated with mood regulation. They concluded that AVE therapy may be a viable nonmedication therapeutic intervention for individuals with mild to moderate depression.
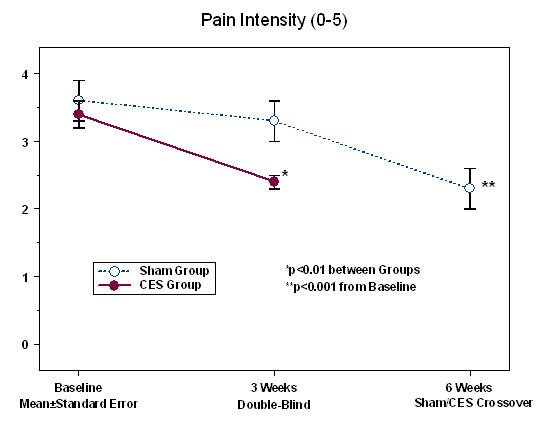
Treatment of Insomnia: A small 2014 pilot study by Hsin-Yi Tang and colleagues at the University of Washington in Seattle tested the efficacy of low frequency (8 Hz descending to 1 Hz) audio-visual EEG entrainment for the promotion of sleep in individuals with chronic pain. This study showed that 30-minutes of low frequency audio-visual stimulation nightly at bedtime over a period of one month significantly reduced insomnia as well as experienced pain intensity in all patients completing the study. A one month post-treatment follow-up showed that positive effects on sleep and pain were well maintained. It appears that low frequency AVE enhanced low frequency brain waves while reducing high frequency brain activity resulting in decreased hyperarousal and improved sleep with consequent reduction in experienced pain and pain-related dysfunction.
AVE is a promising and inexpensive self-care intervention option for individuals experiencing insomnia that uses preprogrammed light and sound patterns to potentiate sleep-related EEG activity in the 0-8 Hz delta and theta frequency ranges.
Selected Readings:
Anderson, D. (1989). The treatment of migraine with variable frequncy photic stimulation. Headache, 29: 154-155.
Berg, K., Mueller, H., Seibel, D., et al. (1999). Outcome of medical methods, audio-visual entrainment, and nutritional supplementation in the treatment of fibromyalgia. Unpublished manuscript available from Mind Alive Inc., Edmonton, Alberta, Canada.
Berg, K., Siever, D. (2009). A controlled comparison of audio-visual entrainment for treating Seasonal Affective Disorder. Journal of Neurotherapy, 13(3):166-175.
Budzynski, T., Budzynski, H., Sherlin, L. & Tang, H. (2011). Audio-visual stimulation: Research and clinical practice. In J.berger & G. Turow (Eds.). Music, Science, and the Rhythmic Brain (pp.137-153). New York, NY: Routledge.
Cantor, D. & Stevens, E. (2009). QEEG correlates of auditory-visual entrainment treatment efficacy of refractory depression. Journal of Neurotherapy, 13(2): 100-108.
Collura, T. (2001). Application of repetitive visual stimulation to EEG neurofeedback protocols. Journal of Neurotherapy, 6(1): 47-70.
Collura, T., Siever, D. (2009). AVE in relation to mental health and EEG. In J.R. Evans & A. Abarbanel (Eds). Quantitative Electroencephalography and Neurofeedback, Second Edition. (pp.155-183). San Diego, CA: Academic Press.
Frederick, J., Lubar, J., Rasey, H., et al. (1999). Effects of 18,5 Hz audiovisual stimulation on EEG amplitude at the vertex. Journal of Neurotherapy, 3(3): 23-27.
Frederick, J., Timmerman, D., Russell, H., et al. (2005). EEG coherence effects of audio-visual stimulation (AVS) at dominant and twice dominant alpha frequency. Journal of Neurotherapy, 8(4): 25-42.
Gagnon, C., Boersma, F. (1992). The use of repetitive audio-visual entrainment in the management of chronic pain. Medical Hypnoanalysis Journal, 7: 462-468.
Haung, T., Charyton, C. (2008). A comprehensive review of the psychophysiological effects of brainwave entrainment. Alternative Therapies in Health & Medicine, 14(5).
Joyce, M., Siever, D. (2000). Audio-visual entrainment program as a treatment for behavior disorders in a school setting. Journal of Neurotherapy, 4(2): 9-15.
Ossebaard, H. (2000). Stress reduction by technology? An experimental study into the effects of brainmachines on burnout and state anxiety. Applied Psychophysiology & Biofeedback, 26: 93-101.
Patrick, G. (1996). Improved neuronal regulation in ADHD: An application of fifteen sessions of photic-driven EEG neurotherapy. Journal of Neurotherapy, 1(1): 27-36.
Rosenfeld, J., Reinhart, A., Srivastava, S. (1997). The effects of alpha (10 Hz) and beta (22 Hz) entrainment stimulation on the alpha and beta EEG bands: Individual differences are critical to prediction of effects. Applied Psychophysiology & Biofeedback, 22: 3-20.
Siever, D. (2007). Audiovisual entrainment: history, physiology and clinical studies. In J.R. Evans (Ed). Handbook of Neurofeedback: Dynamics and Clinical Applications. (Chapter 7, pp.155-183). Binghampton, NY: Haworth Medical Press.
Teplan, M., Krakovska, A., Stolc, S. (2006). EEG responses to long-term audio-visual stimulation. International Journal of Psychophysiology, 59(2): 81-90.
Teplan, M., Krakovska, A., Stolc, S. (2011). Direct effects of audio-visual stimulation on EEG. Computer Methods & Programs in Biomedicine, 102(1): 17-24.

For more information on audiovisual entrainment and purchasing AVE devices in Edmonton
or anywhere else in the world by internet... GOTO: www.mindalive.com
Bio-Acoustical Utilization Device (BAUD) Therapy
Disrupting the neural pathways to unwanted emotions.
The BAUD, or Bio-Acoustical Utilization Device, is a relatively new and powerful neurotherapy tool for personal change. Developed in 2003 by Dr. G. Frank Lawlis, an American pioneer in medical psychology and Clinic Director of the Lawlis-Peavey Clinic in Dallas, Texas, the BAUD is a unique hand-held, FDA-cleared, Class II neurostimulation device that uses acoustical/sound energy to disrupt and rewire neural pathways that have become overly sensitive and excitable from a sudden traumatic or highly stressful event or by persistent repetition as in the case of chronic pain or stress or compulsive behaviors. The BAUD process can be thought of as "de-training" the brain or "resetting the fear switch".
Over the last decade, many hundreds of therapists world-wide have reported that the BAUD can be used with great effect in the treatment of emotional issues such as fear, anxiety and anger; post-traumatic stress and phobias; psychological suffering from chronic pain; as well as strong compulsions, urges, cravings and appetites -- literally any unwanted feeling or sensation.
The Brain: Source of All Problems
There are powerful energies at work in the brain that help drive reactions and compulsions. It has long been known that stress plays a major role in all sorts of problems: overeating, anxiety, depressed mood, anger reactions, and even addictions. Stress will magnify your problem when it pushes you to react to a situation the same way, over and over again. Every time you reach for a snack to calm down, or let yourself dwell on your fears, you actually create and strengthen neural pathways in the brain. These pathways are kind of like ruts in a well-worn road. Once laid down, you can follow the tracks easily, but it is extremely difficult to pull yourself out of them. So it becomes harder and harder to resist these unwanted urges or reactions. It becomes a vicious circle. Each time you react, the ruts just get deeper. Eventually your brain gets stuck in the “on” position and your unwanted habit becomes a constant nagging urge that seems impossible to resist or get rid of!
Changing these negative feelings or reactions is extremely difficult since the source is largely unconscious. You don’t fully understand it; you can only feel it as an intense emotional pressure that expresses as an out-of-control appetite or a mind obsessed with worry or overwhelmed with fear or anger. Until the source of this stress is relieved, the neural pathway within the brain stays active and the unwanted urge or feeling remains strong.
How does the BAUD work?
The Baud is designed to emit sound frequencies that have commonly been found to resonate with the human body. The user simply tunes the BAUD, and these frequencies mix to create binaural pulses that initially match the brain frequency of the unwanted neural pattern and then interfere with the brain pattern and disrupt it.
The basic BAUD protocol is quite simple and used with every type of problem…
1. First, you activate your target issue by focusing your attention on it. Try and identify the location or area in your body where you actually feel your problem and allow yourself to fully experience it. For example, your anxiety about driving in busy city traffic and the feeling of nausea in the pit of your stomach that arises. This activates the corresponding areas of the brain involved in this pattern of worrying thoughts, images, emotions, and physical feelings.
Very Important Point... You must feel it to heal it.
2. Next, you switch the BAUD on and balance the sound in your earphones by turning the right and left Volume control knobs (lower two knobs) on the BAUD until the sound you hear is moderately loud (not deafening) and perfectly equal in both ears so that it seems centred in your head. The BAUD audio tone is a full square wave that is typically perceived as unpleasant or even somewhat irritating and designed to elicit an amygdala-based emotional arousal.
3. Next, you slowly tune the Pitch or Arousal frequency of the BAUD (upper right pitch knob) until you feel the sound connect to either the body location of your problem— or, more importantly with your emotions themselves. Usually you will experience both at once and a noticeable intensification of your target feeling (e.g., anxiety, craving, pain, etc.). This is true whether the target feeling is emotional, an impulse or craving, or a physical sensation such as pain.
4. After you have found the proper Pitch setting, continue to focus your attention on your problem feelings as you slowly turn the upper left Disruptor knob on the BAUD until you reach a place where the sound seems to neutralize or reduce your problem feeling (e.g., anxiety) you are focusing on. This indicates you are affecting the targeted neural areas of the brain. In the same way that you might tune-in a radio station, you may turn the Disruptor knob past this point and then go back, to confirm this is the strongest neutralizing point. The BAUD will be effective over a certain frequency range, not just one point, but tuning as precisely as you can will help maximize results.
5. Lastly, you should continue to try to keep the target problem active by continually bringing it to mind and, therefore, keeping the neural activity going in the targeted brain areas. Try and do this for anywhere from 10 to 20 minutes after you have found the Disruptor frequency that reduces the targeted feeling before turning the BAUD off and ending the session. Allowing yourself to settle into slow (e.g., one complete breath every 10-11 seconds), smooth and rhythmic breathing from the belly during these last 10-20 minutes of the BAUD session can add significantly to the effectiveness of the response.
The BAUD works most strongly on these active brain areas, and in a short time, there is usually a marked reduction in the problem’s level of intensity. The key to successfully using the BAUD is to awaken and focus on the feeling state that is associated with your problem-- You must feel it to heal it. This includes both the physical and emotional feelings connected with your problem. A typical BAUD session may last from 15-30 minutes.
While most BAUD users will experience a distinct and meaningful reduction in the intensity of their problem emotions/feelings with the very first BAUD session, it usually requires a number of repeated sessions targeting the same problem feelings to obtain complete relief. Generally the more severe and chronic the problem, the more treatments will be required.
Based on results seen to date, it is believed that the BAUD is able to stimulate a parasympathetic response that brings the targeted neural circuits out of an aroused, or hyperactive state. BAUD effects are not just a momentary distraction. Quantitative EEG, LORETA, and fMRI brain imaging shows positive neural functional changes both during BAUD sessions and persisting long after. Patients also report enduring relief following successful BAUD therapy.
New research is showing that BAUD works by a unique acoustical stimulation that inhibits the reconsolidation of overstimulated (i.e., problem) neural pathways in the limibic system (i.e., subcortical region for emotional processing) -- especially in the amygdala (i.e., fear center) -- while they are activated. It appears to safely stimulate a neural "reset" of the sensitized neural circuit in the brain.
See Garzione, J. & Bruursema, R. (2016). New technique shows promise as adjunct in chronic pain management. Practical Pain Management.com January/February 2016.
See Orlando Sentinal article of August 29, 2015 "Veteran's With PTSD Praise BAUD Audio Therapy...
http://www.orlandosentinel.com/news/os-ap-ptsd-audio-therapy-20150829-story.html
Results are typically experienced rapidly within and immediately after a session, with most people reporting as much as 50% to 70% reduction in experienced pain or anxiety and often feeling profound improvement in stress-driven issues that may have plagued them for years, even a lifetime. While individual results will vary, most BAUD users experience a degree of relief from one session that will last anywhere from a few hours to several days or longer.
What problems can the BAUD treat?
The BAUD is showing itself effective in treating many different emotional and physical symptoms. Dr. Lawlis has personally used it well over a thousand patients over the last few years at the Lawlis-Peavey Clinic to treat a broad range of emotional problems, physical cravings, and pain, and many other therapists in both the United States and Canada have reported successfully treating their patients for many different emotional issues with the BAUD. Whether treating emotional or physical problems, the BAUD protocol is the same and has shown itself successful in substantially alleviating emotional issues in nearly 4 out 5 people. Some of the problems that have been successfully treated are…
- anxiety and specific fears or phobias
- anger and guilt
- post-traumatic emotional distress
- obsessions and compulsions
- chronic pain and pain-related emotional distress
- strong cravings for food, alcohol, tobacco, drugs
A recent survey of physicians, psychiatrists, and psychologists using the BAUD in 14 countries found that 96% of the surveyed professionals reported positive results with the BAUD; with 76% rating these results as good to excellent -- meaning that up to 70% of the problem symptom was reduced after the first treatment session.
Treatment of anxiety with the BAUD: A pilot study
M. Polachek & J. Schwartz
Center for Discovery
Wittier, CA. 2013
Sixty (19 male + 41 female) teenaged (13-17 y.o.) individuals being treated within a private psychological treatment facility were randomly assigned to two groups. Group 1 received the standard group and individual psychotherapy plus pharmacotherapy treatment while Group 2 recieved BAUD therapy sessions along with the standard treatment Patient anxiety was assessed both rior to beginning treatment and two weeks after completing treatment. The group with the added BAUD therapy showed significant reductions in measured anxiety whereas the group receiving the standard therapy only showed no significant reduction in anxiety.
To see a brief video segment from The Doctors television show on using the BAUD to treat various forms of craving, GOTO: http://www.thedoctorstv.com/videolib/init/5583
To see a YouTube video segment from the Dr. Phil Show that discusses the use of the BAUD device to reduce food craving in the treatment of overeating, GOTO: http://www.youtube.com/watch?v=E8YHDMKg1wA
To see a YouTube video with Dr. Frank Lawliss, the Director of the Peavey Neurotherapy Center in Dallas and the inventor of the BAUD device, discussing the treatment of depression and the use of the BAUD device in treating depression, GOTO: http://www.youtube.com/watch?v=gnYn633vkdg
To see YouTube videos of Sue Shipman of the River Source Drug and Alcohol Treatment Centers talk about using the BAUD in the treatment of drug addictions and anxiety,
GOTO: http://www.youtube.com/watch?v=Nb3cqt0wHwA
http://www.youtube.com/watch?v=6oS10xd0rH8
http://www.youtube.com/watch?v=SOooE1xqAis
How safe is the BAUD?
The BAUD is registered as a Class II device with the United States Food and Drug Administration (FDA) and is recognized as safe. Since its initial development and trails at the Peavy Neurotherapy Centre in Dallas, Texas, in 2003, the BAUD has been used to treat many thousands of individuals in 16 countries without a single report of any serious adverse effects or lasting side-effects. Although relatively rare, the most commonly reported adverse events have been mild and time-limited headaches, temporary dizziness and feelings of mild nausea, and experiencing unwanted strong feelings without obtaining any reduction within the session. As well, some individuals who are especially sound sensitive may find the BAUD sound too unpleasant and/or may experience some continued sensitivity to environmental sounds or hear non-existent sounds (e.g., ringing or buzzing in the ears, humming, water running, etc.) for a brief period after using the BAUD. The BAUD device is not recommended for use by individuals suffering from tinnitus.
The BAUD is available from Insight NeuroSystems LLC, 15954 Mur-Len, Unit 125, Olathe, Kansas 66062, USA. www.mybaud.com Also GOTO: www.baudenergetics.com www.baudtherapy.com www.baud-biofeedback.com
Cranial Electrotherapy Stimulation (CES)
- CES is a US Food and Drug Administration-approved, prescriptive, noninvasive,
electromedical treatment that has been shown to significantly decrease anxiety,
insomnia, and depression.
- Side effects from CES are quite mild and self-limiting and experienced by less
than 1% of users and include vertigo, skin irritation at electrode sites, and headaches.
- While CES treatment effects are cumulative and major symptom relief may
require regular use over a few weeks, many patients experience at least some
improvement in their symptoms after the first one or two treatments. Anxiety
responds most readily to CES and most patients experience noticably reduced
general anxiety within a week or less. Depression can take up to 3-4 weeks
for initial response. Insomnia varies widely with some individuals having
improved sleep immediately and others not seeing improved sleep until more
than 1-2 months into treatment.
- A trial treatment in the clinician's office can identify those individuals who readily
respond to CES treatment. CES can also be used during psychotherapy
sessions and with medications, hypnosis, and biofeedback to decrease patient anxiety.
- CES is cost-effective compared with drug therapy or other devices used in
psychiatry for problems with anxiety, insomnia and depression and it is very easy
to use in both clinical and home settings.
Cranial Electrotherapy Stimulation (CES), also known as Cranial Electrical Stimulation, Cranial Electrostimulation Therapy (CET), or Transcranial Alternating Current Stimulation (tACS) has been categorized as a form of alternative medicine broadly called "Electromedicine", that treats both physical and psychological conditions with varying levels of electrical current. CES is the application of a very low level of alternating (AC) current (usually less than 4 milliamperes) to the brain by means of earclip electrodes attached to the earlobes or electrodes taped to the bony mastoid process just behind each ear or to various other locations on the skull for treatment of anxiety, depression, insomnia, chronic pain, and substance addictions. A similar form of low amperage alternating current electrostimulation is called Microcurrent Electrotherapy (MET) or microTENS and is applied through electrodes taped to the skin over muscles and is used to treat muscle tension and pain by massage therapists and physical therapists.
CES was approved in the United States by the Federal Drug Administration (FDA) in 1979 within a category for medical devices using microcurrent levels of electrical stimulation across the head via transcutaneous electrodes for the treatment of anxiety, depression, and insomnia and is commercially available in the United States for personal use with a prescription from a health care provider. Canadians have no such restrictions on the personal use of these devices at this time.

CES was originally developed in the Soviet Union in 1949, its primary focus being the treatment of sleep disorders— hence, its original designation as “electrosleep”. The treatment of insomnia was soon overshadowed by psychiatric applications for anxiety and depression.
The treatment of anxiety and depression with CES began in the United States in the early 1960s and CES is currently routinely prescribed by thousands physicians and mental health practitioners in the US and Canada for a variety of brain-related psychiatric conditions, although it has yet to achieve full acceptance as a mainstream medical treatment. This is probably because sufficient information has not been made available to the majority of medical practitioners regarding the safety and efficacy of CES and the fact that the pharmaceutical industry spends a great deal of money every year promoting the use of medications instead of alternative therapies as CES.
While medicine and the general public have largely been conditioned since the 1950s to believe that there is a pharmacological solution for every symptom or disease, the brain is much more than just a chemistry lab and it has become increasingly apparent that treatment of mental disorders such as anxiety and depression with drugs alone is insufficient. An old approach that has become new again, electromedicine focuses on impacting mental and physical health through the electrical nature of the body and brain.
While there are over 170 published scientific research studies on the use of CES. The overwhelming majority of these studies support the safety and efficacy of CES in the treatment of a number of psychological disorders; particularly anxiety, depression and insomnia. Yet the majority of Canadian physicians in general medical practice are simply unaware of them. Unlike pharmaceuticals, there is no large industry promoting CES to physicians.
While CES is FDA-approved solely for the treatment of anxiety, depression and insomnia, there is scientific data showing promise in the treatment of other conditions such as chronic pain, tension/migraine headaches, fibromyalgia, and substance dependencies (i.e., may reduce symptoms associated with alcohol, drug or tobacco withdrawal), as well as for calming agitated and aggressive patients with neuropsychiatric conditions. The research to date is strongest for CES as a treatment for generalized anxiety and insomnia.

Left to right, Mind Alive Inc. Oasis Pro device, Alpha-Stim 100 device, Neurofitness LLC CES Ultra device,
and Fisher Wallace Cranial Stimulator
How does CES work?
CES is a relatively simple treatment employing a small, battery-powered device that is similar in size and appearance to transcutaneous electrical nerve stimulators (TENS) devices commonly used in physical therapy for pain relief, but produce very different waveforms at a much lower current level. The CES device sends pulses of very low amperage electricity (i.e., typically 100 microamperes to 4 milliamperes) through thin wires attached to electrodes that are either clipped to the ear lobes or stuck to the skin over the bony prominences just to the front of, or behind each ear. The frequency of the electrical pulses can be adjusted— usually from 0.5 Hz to 100 Hz— depending on the treatment effect desired. The devices of different manufacturers will typically differ in the specific waveforms (e.g., square or sine waves), frequencies and pulse-widths, and maximum amperage of the electrostimulation provided.
CES devices function differently from other biomedical electronics, such as deep brain stimulating electrodes (used to prevent seizures and hand tremors) and heart pacemakers. While those instruments require surgical implantation, CES operates non-invasively. CES is also quite different from electroconvulsive therapy (ECT) or electroshock therapy; a therapeutic modality sometimes used in hospitals to treat severe depression that is not responsive to medication. Where ECT uses a strong, steady (DC), electrical current applied directly to the scalp under anesthesia to cause a controlled brain seizure, CES applies a pulsing AC current that is over a thousand times weaker than ECT to the earlobes or skull to induce changes in the excitability and average firing frequency of brain neurons but does not cause resting neurons to actually fire. CES is painless and non-invasive and many CES devices are designed for consumer use in-home.
As with most medications for psychological problems, the actual mechanism by which CES works remains unclear but CES is increasingly being viewed as an adaptogen, in that itreduces stress that underpins many emotional disorders. Research to date suggests a number of possible mechanisms of action, including direct action on the brain at the level of the limbic system, the reticular activating system and the hypothalamus, increased release of various neurotransmitters (e.g., serotonin, dopamine, norepinephrine) and endorphins in the brain, increased parasympathetic nervous system dominance via the vagus nerve, and changes in blood flow and the electrical rhythms (EEG) of the brain. Rat studies have reported a three-fold increase in endorphin levels with only a single relatively brief exposure to CES. Some researchers have reported rapid increases in serotonin levels in humans, a brain neurotransmitter associated with relaxation and calmness, and decreases in cortisol, one of the primary stress-related hormones in patients treated with CES. As well, CES is known to increase levels of the brain neurotransmitters norepinephrine and dopamine, both associated with alertness and feelings of pleasure. Interestingly, serotonin, norepinephrine and dopamine are the same neurotransmitters that most antidepressant medications attempt to activate. MRI studies suggest the CES may reduce activity in brain areas associated with pain processing such as the insula, cingulate gyrus, and prefrontal cortex, as well as affecting brain stem nuclei that radiate widely through the central nervous system and affect all the systems inportant in mood and anxiety disorders.
Animal studies indicate that 40-45% of the CES current actually enters the brain, with the highest levels of current recorded in the thalamus-- the primary communication link between deep brain regions and the outer cortex. The thalamus is also a brain structure that appears to pay an important role in the pathophysiology of anxiety and in transmitting pain-related signals from the body up through the brain to the cortex. Medications that are effective in reducing anxiety and some forms of pain appear to reduce activity in the thalamus and one theory of CES suggests that the alternating current stimulation interferes with ongoing brain wave oscillations by introducing cortical noise.
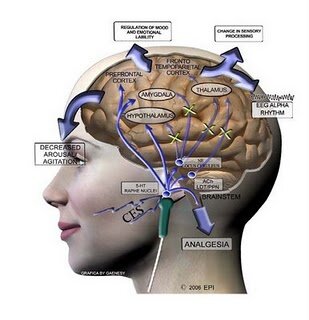
CES has also been demonstrated to increase and slow brain electrical patterns known as “alpha rhythms”. Increases in the amount and power of alpha waves in the brain are associated with meditation and increased feelings of relaxation and calm focus. As well, Kennerly (2006) also reported a reduction in very slow delta and high frequency beta brainwaves. Reduction in high frequency brain activity between 20-30 Hz correlates with reductions in anxiety, ruminative thought, and obsessive-compulsive behaviour (Demos, 2005). Using QEEG and LORETA analysis techniques, Kennerly also reported that the electrical impulses generated by CES reached all cortical and sub-cortical areas of the brain.
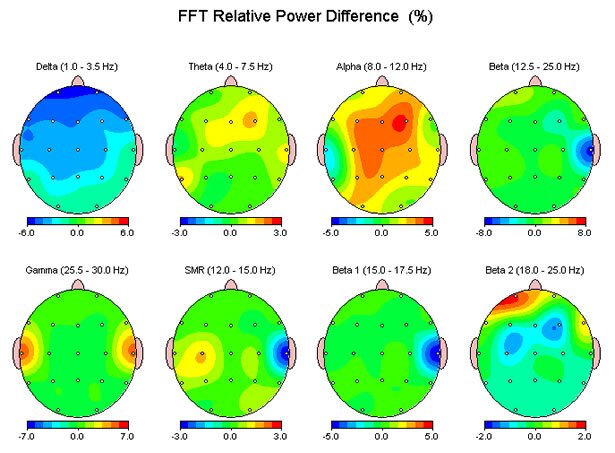
The figure above shows the change in EEG relative power across the clinical frequency bands for an individual following a single 30-minute stimulation session using an Alpha-Stim 100 device. Note the increases in 8-12 Hz Alpha centrally and Gamma in the auditory cortices.
A recently published study by Dr. Jamie Feusner at the School of Medicine UCLA (Feusner, et al., 2012), used functional MRI scanning to study the effects of 0.5 Hz ans 100 Hz CES from the earlobes. The results from this study suggest that CES causes cortical brain deactivation in midline prefrontal and parietal regions and, in addition, 100 Hz CES (but not 0.5 Hz) significantly altered functional connectivity within the "default mode network". Moreover, cortical deactivation patterns differed from those associated with current intensity, suggesting that cortical deactivation may depend more on the frequency of stimulation than on its intensity.
To view a YouTube video of David Siever, CEO of Mind Alive Inc. lecturing on Cranial Electrostimulation at the March 2011 Annual Conference of the Association for Applied Psychophysiology and Biofeedback in New Orleans, GOTO: http://www.youtube.com/watch?v=PDK68rpM7k0&feature=related
What does CES feel like?
Applied to the ear lobes or to the mastoid, just behind the ear, CES causes the patient to experience nothing more than a faint tingling sensation. As the treatment continues, the tingling tends to disappear and most patients begin to feel less anxious, less distressed, and more relaxed and, yet, mentally alert and focused. Patients with positive response to CES generally sleep better and report improved concentration, increased learning abilities, enhanced recall and a heightened state of well-being after one or a series of CES treatments. Most people can resume normal activities immediately after a CES session. Others may experience a mild euphoric feeling, or a state of deep relaxation that may temporarily and minimally impair their mental and/or physical abilities for the performance of potentially hazardous tasks, such as motor vehicle operation. In some cases, this may last for up to several hours after treatment. Users may do other things during treatment such as read, watch TV, engage in conversation, or work on a computer.
CAUTIONARY NOTE: Until you have experienced CES for yourself and are certain of how you will react to treatment, it is best that you do not operate a motor vehicle or other motorized equipment or engage in potentially hazardous activities immediately after treatment.
Most patients are left feeling relaxed and alert after a CES session— in what psychologists call an “alpha state”. This state differs from pharmaceutical treatments in that people report feeling that their bodies are lighter and more relaxed and their minds more alert and clear. The results tend to be cumulative and long lasting.
TESTIMONIAL: Sgt. Michael Conte, who served in Iraq for the U.S. Army, uses the Alpha Stim cranial electrotherapy stimulator, to treat symptoms of a minor brain injury suffered in 2007 following an IED explosion. "During the long process of my treatment and some of my comrades' treatment we have been given nuero stimulation and nuerofeedback. One of the devices we are authorized to buy and use is the Alpha-Stim. For some people it increases mood, for others it's a pep like caffeine, for some it calms them down enough to sleep, for me it clears the fog which I consistently live in due to my injury. The results last most of the day for me and allow me to continue my job with little loss of function."
What are the adverse effects of CES?
Unlike the United States, where CES devices are sold by prescription only, there are no specific restrictions in Canada on the purchase or home-use of CES devices by the consumer. Canada Health does not recognize CES for the treatment of any medical condition and, therefore, no commercial retailer of CES devices can make any direct claims for its effectiveness in treating any medical condition. That said, no one is actually proscribed from using a CES device to treat any condition they wish.
CES has a proven track record of safety, especially in comparison to alternative pharmaceutical treatments for the same conditions. No medically serious adverse effect has ever been reported from using CES; there have been no reports of lasting adverse effects, significant side-effects, or any serious contraindications to CES treatment. CES treatment has not been shown to interact negatively with any medications and may be used adjunctively with psychoactive medications.
Adverse-effects reported in research to date have been mild and time-limited and include... dizziness/nausea (<1%), skin irritation or mild electrode burns (<1%), and headaches (<1%). In rare cases, CES may trigger temporary paradoxical reactions of hyperexicitability, increased anxiety, and sleep disturbances. There have been a few reports of persons suffering from posttraumatic stress disorder (PTSD) experiencing more vivid and sometimes disturbing dreams at night. This latter adverse-effect may be ameliorated by not using CES too close to bedtime.
Headaches and vertigo side-effects are usually associated with the current being set too high for the individual and these effects generally resolve when the current is reduced or within minutes to hours following treatment. Irritation at the electrode site can be avoided by ensuring good electrical contact and moving the electrodes around slightly during treatments.
That said, labeling of CES devices contains precautions seen on all electromedical devices against use by pregnant women and persons with implanted medical devices such as cardiac pacemakers. Due to the relaxing effect of CES treatment, patients are also cautioned in the use of hazardous machinery or driving during or immediately after CES treatment. Despite the known safety of CES, it is advisable to only use CES to treat clinical conditions under the direction and supervision of a health professional and to keep your primary care physician informed.
What is the evidence for the effectiveness of CES?
Research studies of CES that been published to date reveal significant changes associated with relaxation responses such as reduced muscle tension, positive changes in brain wave activity, increased vasodilation, reductions in gastric acid output, and reductions in blood pressure, pulse, respiration, and heart rate. CES research has also shown significant reductions in clinical depression (Gilula & Kirsch, 2005), anxiety (Klawansky, et al., 1995) and specific fibromyalgia symptoms (Lichtbroun, et al., 2001; Taylor, et al., 2011).
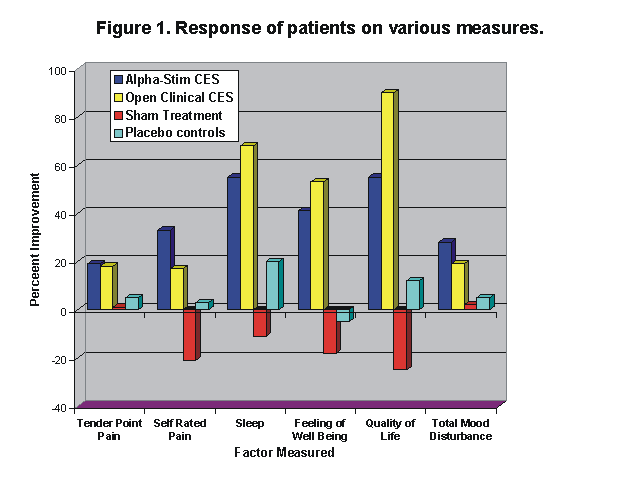
More than 25 clinical research studies examining the efficacy of CES for the treatment of depression have been published, with over 80% of these studies reporting significant clinical improvements in the symptoms of depression (Gilula & Kirsch, 2005).
A recent meta-analysis of 22 placebo-controlled CES research studies involving a total of 1075 patients found that the average treatment effect beyond that attributable to placebo was 57% (Gilula & Kirsch, 2005). This compares very favourably with the often claimed 40-60% average treatment effects beyond placebo for antidepressant medications.
The available clinical research on the use of CES to treat mild to moderate depression shows that approximately two-thirds of individuals using CES daily over periods of 2-4 weeks will experience greater than 50% improvement in their symptoms; with about a third of these responders obtaining greater than 75% improvement within 4 weeks.
For the symptoms of anxiety alone, CES results in approximately 75% of users obtaining greater than 50% reduction in their anxiety symptoms.
The book— Cranial Electrotherapy Stimulation— by Dr. R.B. Smith (2007) reviews the results from over 100 studies involving over 4000 subjects and reports that CES is highly effective in the treatment of insomnia, anxiety, depression, drug abuse, anxiety and cognitive dysfunction with an average of 67% of patients reporting significant improvement in their symptoms.
CES has also been shown to improve sleep and memory consolidation during sleep (Born, et al. 2006; published in Nature).
How is CES treatment actually done?
CES devices are quite simple to use, relatively inexpensive (generally about $300-$500) and easily purchased in Canada from a local or internet specialty store without prescription. [In Edmonton, go to Mind Alive Inc.] And while most individuals are capable of doing self-directed CES therapy at home for minor problems, it is best to use CES under the advisement or supervision of a qualified health care professional when treating a more significant mental health condition such as an anxiety disorder, clinical depression, severe insomnia, or chronic pain or associated symptoms. Health care professionals may be able to add other forms of treatment to CES, such as psychotherapy, cognitive-behavioral therapy, stress-management counselling, biofeedback self-regulatory training, or nutritional advice to obtain better results in more complex, chronic, or severe cases.
In all cases, the health care professional will need to see you for at least one or two appointments to assess your condition, evaluate your initial response to the CES treatment within the safe and controlled environment of their office, and show you how to properly use the device. In some cases, the health care practitioner will offer a series of regular CES treatments in his/her office; often in conjunction with other treatments such as counselling or psychotherapy or biofeedback. In still other cases, the health practitioner may provide you with a "rental" CES device for use at home for a specified period of time or will encourage you to purchase your own device for use at home.
While there certainly may be situations in which it is both practical and sensible to include CES treatments as part of regular clinical visits, in most situations it is far more cost-effective for the patient to use CES on a daily basis at home for a period of time and only see the health practitioner in the clinic for follow-up and any other related treatments. Home treatments may need to be performed daily during the first 1 to 3 or 4 weeks and then 2 or 3 times per week during a maintenance phase. The individual can use the CES device as often as needed, as there are no known adverse effects from extended use beyond those rare mild side-effects already identified. Using CES on an "as needed" (prn) basis is especially beneficial for those individuals experiencing posttraumatic stress or anxiety with panic attacks or pain.
In the treatment of anxiety or depressed mood, the research has generally shown that approximately 30-60 minutes of CES daily for periods of 2-4 weeks is effective in significantly alleviating symptoms for extended periods of time. However, some people find that their symptoms will slowly return over time and that they benefit from one or more follow-up series of CES treatments. Others find that regular use of the CES device for 30-60 minutes a few times a week will effectively maintain treatment gains indefinitely or successfully manage chronic anxiety, depressed mood, insomnia, pain, or stress.
What results can I expect?
Individual results may vary dependent on a variety of factors, including the severity and chronicity of the condition or symptoms being treated, what medications are being taken (if any), the presence of other concurrent medical factors or comorbid conditions and, ultimately, your level of motivation. Some disorders can be successfully treated in 10-20 sessions; others require more extensive treatment. In the case of more chronic conditions, CES may be required on a regular basis indefinitely to manage symptoms.
For those patients with more severe acute or chronic conditions there may be extra benefit to combining CES with counselling or psychotherapy as well as other forms of neurotherapy such as Audio Visual Entrainment (AVE) therapy or certain biofeedback therapies such as Heart Rate Variability (HRV) or Electrodermal (ED) biofeedback.
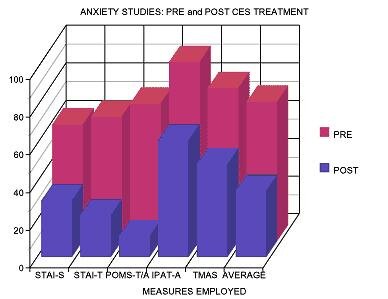
The graphs above show data from studies conducted by Neurofitness LLC using the CES Ultra device.
To view a brief YouTube video on the use of the AlphaStim CES device to treat depression, GOTO: http://www.youtube.com/watch?v=tsaeTlzvHwk&feature=related
For more information on purchasing CES devices…
Mind Alive Inc. in Edmonton manufactures and sells a number of different CES devices, including the Oasis Pro device GOTO: www.mindalive.com
Optimum Health in Edmonton and Natural Pain Products Inc. in Calgary are Canadian distributors for the AlphaStim 100 Microcurrent Stimulator GOTO: www.optimumhealth.ca www.naturalpainproducts.ca
Neurofitness LLC in Seattle manufactures the CES Ultra device GOTO: www.cesultra.com
The Canadian-made "Harmonizer" CES device can be purchased from: www.harmonizer.com
Other CES devices can be purchased in Canada from: www.soundhealthproducts.com and www.fisherwallace.ca
Selected Bibliography
Amr, M., El-Wasify, M., Elmaadawi, A., et al., (2013). Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. Journal of ECT, 29(2): e31-e32.
Anderson, J., Kebaish, S., Lewis, J., et al. (2014). Effects of cranial electrical stimulation on activity in regions of the basal ganglia in individuals with fibromyalgia. Journal of Alternative & Complementary Medicine, 20(3):206-207.
Barclay, T., Barclay, R. (2014). A clinical trial of cranial electrostimulation for anxiety and co-morbid depression. Journal of Affective Disorders, 164: 171-177.
Bracciano, A., Chang, W-P., Kokesh, S., et al. (2012). Cranial electrotherapy stimulation in the treatment of post-traumatic stress disorder: A pilot study of two military veterans. Journal of Neurotherapy, 16: 60-69.
Bystritsky, A., Kerwin, L., Feusner, J. (2008). A pilot study of cranial electrotherapy stimulation for generalized anxiety disorder. Journal of Clinical Psychitary, 69(3): 412-417.
Childs, A., Price, L. (2007). Cranial electrotherapy reduces aggression in violent neuropsychiatric patients. Primary Psychiatry, 14(3): 50-56.
Datta, A., Dmochowski, J., Guleyupoglu, B. et al. (2013). Cranial electrostimulation (CES) and transcranial pulsed current stimulation (tPCS): A computer-based high-resolution modeling study. NeuroImage, 65: 280-287.
Demos, J. (2005). Getting Started With Neurofeedback. New York, NY: Norton & Co.
Feusner, J., Madsen, S., Moody, T., et al. (2012). Effects of cranial electrotherapy stimulation (CES) on resting state brain activity. Brain & Behavior, (Open Access doi: 10.1002/brb3.45).
Gilula, M., Kirsch, D. (2005). Cranial electrotherapy stimulation review: A safer alternative to psychopharmaceuticals in the treatment of depression. Journal of Neurotherapy, 9(2): 7-26.
Holubec, J. (2009). Cumulative response from cranial electrostimulation (CES) for pain. Practical Pain Management, November/December 2009.
Kennerly, R. (2006). Changes in quantitative EEG and low resolution tomography following cranial electrotherapy stimulation. Unpublished doctoral dissertation, University of North Texas, Denton, TX.
Kirsch, D. (2006). Why electromedicine? Practical Pain Management, 7(6): 52-54.
Kirsch, D., Gilula, M. (2007). Cranial electrotherapy Stimulation in the Treatment of Insomnia: A Review and Meta-Analysis. Practical Pain Management, October 2007, pp. 28-37.
Kirsch, D., Gilula, M. (2007). CES in the treatment of anxiety disorders. Part 1 & 2. Practical Pain Management, 7(4): 22-39.
Kirsch, D., Gilula, M. (2007). CES in the treatment of anxiety depression. Part 1 & 2. Practical Pain Management, 7(3 & 2): 32-41.
Kirsch, D., Nichols, F. (2013). Cranial electrostimulation for the treatment of anxiety, depression, and insomnia. Psychiatric Clinics of North America, 36: 169-175.
Klawansky, S., Yeung, A., Berkey, C., Shah, N., et al. (1995). Meta-analysis of Randomized Controlled Trials of Cranial Electrostimulation: Efficacy in the Treatment of Selected Psychological and Physiological Conditions. Journal of Nervous and Mental Diseases, 183(7):478-484.
Lande, G., Gragnani, C. (2013). Efficacy of cranial electrical stimulation for the treatment of insomnia: A randomized pilot study. Complementary Therapies in Medicine, 21: 8-13.
Lichtbroun, A., Raice, M., Smith, R. (2001). The treatment of fibromyalgia with cranial electrical stimulation. Journal of Clinical Rheumatology, 7(2): 72-78.
Polonia, R. (2013). tDCS and resting state fMRI. Clinical Neurophysiology, 124(10):e47.
Scherder, E., Knol, D., van Someren, E., etal. (2003). Effects of low frequency cranial electrostimulation on the rest-activity rhythm and salivary cortisol in Alzheimer's disease. Neurorehabilitation & Neural Repair, 17(2): 101-108.
Schroeder, M.J., Barr, R.E. (2001). Quantitative Analysis of Electroencephalogram During Cranial Electrotherapy Stimulation. Clinical Neurophysiology, 112(11):2075-2083.
Smith. R. (2007). Cranial Electrotherapy Stimulation - Its First Fifty Years, Plus Three. A Monograph.
Smith, R., Tiber, A., Marshall, J. (1994). The use of cranial electrotherapy stimulation in the treatment of closed head injured patients. Brain Injury, 8(4): 357-361.
Taylor, A., Anderson, J., Riedel, S., Lewis, J., et al. (2011). Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Management Nursing, 12(1): 1-9.
Taylor, A., Anderson, J., Riedel, S., Lewis, J., Bourguignon, C. (2013). A randomized, controlled, double-blind pilot study of the effects of cranial electrical stimulation on activity in brain pain processing regions in individuals with fibromyalgia. Explore 2013, 9: 32-40.
Transcranial Direct-Current Stimulation (tDCS)
A non-invasive and safe neurostimulation therapy for the treatment of
depression, obsessive-compulsive disorder, migraine, tinnitus, and chronic pain.
Transcranial direct current stimulation (tDCS) is a relatively new form of non-invasive neurostimulation of the brain that is showing great promise as a safe treatment modality for a variety of clinical conditions including major depression, bipolar disorders, obsessive-compulsive disorder, migraine, tinnitus, as well as central and neuropathic chronic pain disorders. TDCS has also been used to relieve the symptoms of narcotic withdrawal and reduce cravings. There is also some limited evidence that tDCS can be used to increase frontal lobe functioning and reduce impulsivity and distractibility in persons with attention deficit disorder. TDCS has also been shown to at least temporarily enhance language and mathematical ability, boost verbal and motor skills, and improve learning and memory in healthy people. Finally, there is quite an extensive literature on the use of tDCS within stroke rehabilitation programs to enhance post-stroke motor function re-learning. TDCS may hold particular promise when used together with surface EMG biofeedback in neuromuscular rehabilitation following brain injury.
Among the various techniques for non-invasive brain stimulation, tDCS stands out as a relatively simple, inexpensive and user-friendly technology. It involves the application of a weak (i.e., very low amperage), non-alternating (i.e., direct) electrical current to the brain area of interest by means of small electrodes placed on the surface of the scalp to generate an intracerebral current flow that selectively modulates the activity of neurons.
A battery-powered current generator capable of delivering small currents (usually less than 3 milliamperes) is attached to two sponge-based electrodes. The sponge electrodes are soaked in a conductive saline solution, applied over the hair onto the scalp, and held in place by non-conducting elastic bands affixed around the head. Current partially penetrates the skull to change the membrane potentials of neurons in the underlying brain cortex, resulting in real-time neurological effects-- a cascade of neurobiological events at the cellular and molecular levels.
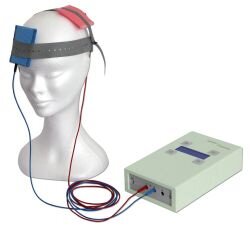
Illustration of transcranial DC stimulation device.
The exact mechanism of tDCS is not clear but extensive neurophysiological research has shown that direct current (DC) electricity penetrates the skull and outer layers of the cortex to modify neuronal cross-membrane resting potentials and thereby influence the level of neuronal excitability and modulate firing rates. Importantly, tDCS only modulates neuronal potential activity and does not actually stimulate action potentials or the firing of resting neurons.
Direct current appears to modulate spontaneous neuronal activity in a polarity-dependent fashion. For example, stimulation with the positive pole (anode) placed over a selected cortical region will increase the exicitability of the underlying neurons while stimulation with the negative pole (cathode) will decrease neuronal exicitability under the electrode. It has been shown that anodal stimulation results in sub-threshold depolarization of the underlying neuronal membranes; increasing their electrotonic potential and making these neurons more easily excited. Cathodal stimulation of the neurons will result in hyperpolarization and a decrease in electrotonic potential of the neuron membrane with a resultant decrease in excitability. The amount of depolarization/polarization and the duration of the change in neuronal excitability is dependent on the current density and duration of the stimulation of the stimulation.
Anodal stimulation is associated with a decrease in GABA neurotransmitter concentration and cathodal stimulation is associated with decrease in both glutamate and GABA. Dopaminergic mechanisms may be involved in NMDA-induced after-effects. Anodal stimulation also increases oxyhemoglobin concentration and associated regional cerebral blood flow in the stimulated area.
In this manner, tDCS may be used to increase cortical brain activity in specific brain areas that are under-aroused or alternatively decrease activity in areas that are overexcited. Research has shown that the effects of tDCS can last for an appreciable amount of time after exposure. Stimulation of less than 5 minutes has little carry over effect once stimulation has stopped, whereas stimulation lasting longer than 20 minutes can have after-effects lasting for a couple of hours or more. Similarly, when stimulation is repeated on a daily basis over a week or two, there is a clear increase in the length of treatment effects often lasting a number of weeks or even months. These long-term effects of tDCS likely involve NMDA-receptor dependent mechanisms. Repeated tDCS is believed to activate neurochemical changes in the affected neuronal matrix similar to those that are known to result in the consolidation of newly learned information into long-term memory.
The behavioral effects of tDCS are primarily dependent on the intensity, duration, location and electrovalence of stimulation. Anodal tDCS of the premotor cortex, for instance, increases the excitability of the ipsilateral motor cortex and inhibition of the contralateral motor areas. More intense or more prolonged or repeated stimulation results in longer lasting after-effects.
tDCS is not ECT
tDCS may sound similar to electroshock or electroconvulsive therapy (ECT) commonly used in psychiatry to treat intractible depression but is actually quite different. ECT is a medical procedure done under anaesthesia that applies electrical currents as much as a thousand times greater than tDCS to the brain for brief periods. ECT induces action potentials in resting neurons throughout the brain resulting in massive neuronal firing and brain seizure. ECT drastically affects the functioning of the entire brain and can result in significant adverse effects, including memory loss and personality changes.
Transcranial DC stimulation on the other hand, uses only a very small electric current that cannot set off a seizure and is far more selective in its effects. No instances of epileptic seizures caused by tDCS have been observed in humans. tDCS primarily influences the area of the cortical brain directly beneath the electrode but can have some effects on deeper brain structures as a function of electrode montage and how current flow is directed during stimulation. tDCS is not associated with any temporary or long-term memory impairments and, as a matter of fact, has been shown to temporarily improve verbal and non-verbal working memory and enhance learning.
tDCS is not TMS
Transcranial direct current stimulation is a relatively unconventional method of stimulating the brain. While this method is gaining interest (over 600 research papers published within the last decade), the most commonly used method of brain stimulation currently used in medicine is transcranial magnetic stimulation (TMS)-- a technique that utilizes an electromagnetic coil held above the scalp over the brain region of interest and applies rapidly changing magnetic fields to induce small electrical currents within the brain. There are two types of TMS: repetitive TMS (rTMS) and single pulse TMS. Both are used in research therapy but effects lasting longer than the stimulation period are only observed in repetitive TMS. Similar to tDCS, an increase or decrease in neuronal activity can be achieved using this technique, but the method of how this is induced is very different. Transcranial direct current stimulation has the two different directions of current that cause the different effects. Increased neuronal activity is induced in repetitive TMS by using a higher frequency and decreased neuronal activity is induced by using a lower frequency.
Both TMS and tDCS are painless and considered safe for human use when properly done. However TMS is far more expensive, difficult to sham, and may need a trained coil holder while tDCS is relatively easy to apply. Transcranial magnetic stimulation causes the neuron's action potentials to fire, resulting in a stronger effect but also greater potential to cause a seizure. Since tDCS only causes increased spontaneous cell firing, it does not have as big as an effect but there is a much smaller chance of causing seizures with tDCS.
One other technique of electrical stimulation that has been used experimentally is called transcranial electrical stimulation, or TES. TES also functions by inducing neuronal change via electrical currents. TES, unlike tDCS, induces an alternating current through the scalp and causes the resting neurons to fire and can be painful to the person receiving the stimulation, so this method is no longer frequently used. TES is related to CES (see above information on CES) but differs in a number of technical ways that can make all the difference in its effects on the brain and how it is experienced by the patient. TES is of great interest to researchers but is far from ready for prime time.
How is tDCS Administered?
The earliest published studies used once-daily tDCS treatments for 5 alternate days; more recent studies used tDCS once-daily for 10 consecutive weekdays or twice daily for 5 consecutive weekdays; and some of the most recent studies have treated patients with as many as 20-30 sessions over periods of four to six weeks. In these published studies all treatments were carried out in a clinical office under the direct supervision of the researcher/therapist or their trained assistants.
Clinically, tDCS is most commonly done in the neurotherapist's office as an "out-patient" treatment consisting of a series of once-daily stimulation sessions, each lasting 20-30 minutes and occuring over a number of consecutive weekdays for periods of a couple of weeks or more.
Unfortunately, such treatment schedules are not very impractical for most patients who are un-hospitalized and still functional within the community or who live some distance from the clinic offering treatment. Certainly for many patients suffering from mild to moderate depression who might benefit from tDCS, such an onerous treatment protocol requiring daily visits to a clinic over periods of a few weeks greatly inhibits access to this form of therapy. But all the research to date has been very consistent in showing that tDCS is a relatively simple and very safe procedure when done using a competently-designed, current self-limiting tDCs device with proper electrodes, and following proper procedures. After initial assessment for suitability and an in-office trial of treatment and some basic training, there is little reason why selected patients could not continue to do tDCS treatment on themselves at home under the supervision and follow-up of a qualified healthcare provider.

Illustration of a patient being treated with tDCS.
With the patient fully awake and comfortably seated in a chair, two 5 cm x 5 cm non-metallic electrically-conductive rubber electrodes are inserted into saline-soaked sponge sleeves and placed on selected locations of the scalp; held in place by elastic headbands. Once the electrodes are properly placed, a tDCS device powered by a 9-volt battery is used to send a steady electrical current of between 1.0 and 2.0 milliAmperes through the electrodes and into the cortex for 20-30 minutes. Dependent on the size of the electrodes used, current densities are maintained at a safe and comfortable level of 30-40 microamps per square-centimeter for the active electrode.
The electrode attached to the positive (anode) pole of the battery will increase neuronal activity in the cortex under it, whereas the electrode attached to the negative pole (cathode) of the battery will inhibit neuronal activity in the cortex under it.
The procedure does not elicit any pain. Persons receiving tDCS generally report nothing more than a mild tingling or itchy feeling from under the electrode during the first few seconds of stimulation; with the sensations usually fading and completely disappearing with about 30 seconds.
Treatment with tDCS is relatively inexpensive, easy to administer, non-invasive, painless, and safe when properly carried out.
To view a YouTube video of a lecture by David Siever, CEO of Mind Alive Inc., on Transcranial DC stimulation at the Association for Applied Psychophysiology & Biofeedback conference in New Orleans in March 2011, GOTO: http://www.youtube.com/watch?v=f3eAU5aXQ9E&feature=related
tDCS is Safe
Numerous studies verify that low-intensity tDCS is safe for humans and that it is linked with only rare, transient, and relatively minor adverse effects. The most common side-effects observed with tDCS are mild tingling while the current is on (71%), moderate transient fatigue (35%), mild itching sensations under the scalp electrodes (30%), slight burning sensation (21%) or mild pain (16%) under the electrodes. Less commonly, some subjects report headache (12%), temporary trouble concentrating (11%), nausea (3%), and temporary sleep disturbance (1%). Reddening or irritation or very mild burning of the skin under the electrodes has also been reported, more commonly with increasing numbers of treatments done closer together in time and at higher amperage or too small electrodes.
Contrast-enhanced MRI and EEG studies have failed to find any pathological changes associated with application of tDCS. That said, no one has as yet studied the brain effects of frequent long-term use beyond about 30-40 treatments taking place over periods of a couple of months or less.
Although patients with a history of seizures are routinely excluded from current tDCS research studies, no instances of epileptic seizures caused by tDCS have been reported in humans and, in a few cases, tDCS has actually been used to treat epilepsy.
The safety of tDCS use in pregnant women and young children has not been investigated and remains unknown. tDCS is not advisable for individuals with implanted medical devices such as pacemakers or cardiostimulators or who have metal mesh or plates under the scalp. tDCS should be applied with caution over scarred areas of the scalp.
With respect to both efficacy and safety, current density is the most important consideration in tDCS use. Even a 1 mA current can cause discomfort and minor skin burns if the electrode used is too small or too dry. It is also essential that the tDCS device is current controlled; automatically adjusting itself with respect to voltage as the skin resistance changes so that the current will always remain the same as set by the practitioner. It is also important that the tDCS device has the capability of turning itself off if the electrode connection is too poor or current flow too high to avoid any possible injury. Absolutely no tDCS device should ever be connected directly to any source of electrical line voltage. Most tDCS devices are completely stand alone and powered through a standard 9-volt dry cell battery.
Most tDCS devices are limited to a maxium output of 2.0 mA and are designed to be used with electrodes that will limit current densities under the electrodes to the range of approximately 20-60 microamps per square centimeter.
While it is entirely possible for nearly anyone to put together a tDCS device from a few inexpensive parts purchased from an electronics supply shop, no one should attempt to do this unless they are properly trained as an electronics engineer and can design circuits that will safely limit currents in the face of fluctuating resistance.
To see a brief YouTube video on transcranial DC stimulation, GOTO: http://www.youtube.com/watch?v=1RMV0yxxMh8
To see a much longer and more academic YouTube video on research with Transcranial DC Stimulation to treat aphasia, GOTO: http://www.youtube.com/watch?v=gSBsQj9HwQg&feature=related
Clinical Applications of tDCS
tDCS has demonstrated some benefit for the relief of chronic pain syndromes including:
- neuropathic pain
- complex regional pain
- fibromyalgia
- migraine headache
- post-stroke pain
- central sensitized pain syndrome
- multiple sclerosis pain
For more information on the use of tDCS in the treatment of chronic pain...
GOTO: tDCS & Pain
For more information on the use of tDCS in the treatment of migraine...
GOTO: tDCS & Migraine
tDCS may provide relief for:
- depression
- epilepsy
- tinnitus
For more information on the use of tDCS in the treatment of depression... GOTO: tDCS & Depression
tDCS has been shown to improve:
- post-stroke functional recovery
- working memory
- cognitive function in both healthy and mildly demented elderly individuals
- obsessive and compulsive rumination
- motor memory
tDCS has also been shown to produce significant changes in cognitive and memory processes with resulting enhancement of learning and task performance.
Selected References
Angelakis, E., Liouta, E. (2011). Transcranial direct current stimulation: Methodology and applications. Journal of Neurotherapy, 15: 337-357.
Antal, A., Kriener, N., Lang, N., et al. (2011). Cathodal transcranial direct current stimulation of visual cortex in the prophylatic treatment of migraine. Cephalalgia, 31(7): 820-828.
Ardolino, G., Bossi, B., Barberieri, S., et al. (2005). Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct-current stimulation of the human brain. Journal of Physiology, 568: 653-663.
Auvichayapat, P., Janyacharoen, T., Rotenberg, A., et al. (2012). Migraine prophylaxis by anodal transcranial direct current stimulation: A randomized, placebo-controlled trial. Journal of the Medical Association of Thailand, 95(8): 1003-1012.
Bationa, R., Pouleta, E., Haesebaerta, F., et al. (2015). Transcranial direct current stimulation (tDCS) in treatment-resistant OCD: An open-label pilot study. Progress in Neuro-Psychopharmacology & Biological Psychiatry.
Biksom, M., Rahman, A., Datta, A. (2012) Computational models of tDCS. Clinical EEG & Neuroscience, 43(3): 176-183.
Berlim, M., Van den Eynde, F., Daskalakis, Z. (2013). Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: A systemic review and meta-analysis of randomized, double-blind and sham-controlled trials. Journal of Psychiatric Research, 47(1): 1-7.
Bolognini, N., Olgiati, E., Maravita, A., et al. (2013). Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain, 154(8): 1274-1280.
Brunoni, A., Adriano, H., Moffa, A., et al., (2017). Trial of electrical direct-current therapy versus escitalopram for depression. New England J. of Medicine, 376: 2523-2533.
Brunoni, A., Ferrucci, R., Bortolomasi, M., et al. (2011). Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Progress in Neuropsychopharmacology & Biological Psychiatry, 35: 96-101.
Brunoni, A., Moffa, A., Sampaio-Junior, B., et al. (2017). Treatment-emergent mania/hypomania during antidepressant treatment with transcranial direct current stimulation (tDCS): A systematic review and meta-analysis. Brain Stimulation, 10:260-262.
Brunoni, A., Valiengo, L., Baccaro, A., et al. (2013). Sertraline vs. electrical current therapy for treating depression clinical study: Results from a factorial, randomized, controlled trial. JAMA Psychiatry, 70:383-391.
Cerruti, C., Schlaug, G. (2009). Anodal transcranial direct current stimulation of the prefrontal cortex enhances complex verbal associative thought. Journal of Cognitive Neuroscience, 21(10): 1980-1987.
Clark, V., Coffman, B., Trumbo, M., et al. (2011). Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: A 1-H Magnetic resonance spectroscopy study. Neuroscience Letters, 500(2011): 67-71.
Concerto, C., Sawah, M., Chusid, E., et al. (2015). Anodal tDCS for chronic pain in the elderly: A pilot study. Aging: Clinical & Experimental Research, July 2015.
d'Urso, G., Brunoni, A., Bartolomeis, A., et al. (2015). Polarity-dependent effects of transcranial direct current stimulation in OCD. Neurocase, May 2015.
d'Urso, G., dell'Osso, B., Ferrucci, R., et al. (2013). Transcranial direct current stimulation (tDCS) for the treatment of major depression: A pooled analysis from the Italian tDCS collaborative group. Clinical Neurophysiology, 124(10): e185.
DaSilva, A., Mendonca, M., Zaghi, S., et al. (2012). tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache, 52(8): 1283-1295.
Dell'Osso, B., Priori, A., Carlo Altamura, A. (2011). Efficacy and Safety of tDCS in Major Depression. Biological Psychiatry, 69: e23-e24.
Dockey, C., Hueckel-Weng, R., Birbaumer, N., et al. (2009). Enhancement of planning ability bt tDCS. Journal of Neuroscience, 29(22): 7271-7277.
Elsner, B., Kugler, J., Pohl, M., et al. (2013). Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke: First results of a systematic Cochrane-Review. Clinical Neurophysiology, 124 (1): e113.
Fagerlund, A., Hansen, O., Aslaksen, P. (2015). Transcranial Direct Current Stimulation as a treatment for patients with fibromyalgia: A randomized control trial. Pain, 156(1): 62-71.
Foerster, B., Nascimento, T., DeBoer, M., et al. (2015). Excitatory and inhibitory brain metabolites as a target of motor cortex tDCS therapy and predictors of its efficacy in fibromyalgia. Arthritis & Rheumatology, 67(2): 576-581.
Hummel, F., Cohen, L. (2005). Drivers of brain plasticity. Current Opinion in Neurology, 18:1-8.
Iyer, M., Mattu, U., Grafman, J. et al. (2006). Safety and cognitive effects of frontal direct-current brain polarization in healthy individuals. Neurology, 64: 872-875.
Jacobson, L., Ezra, A., Berger, U., et al. (2012). Modulating oscillatory brain activity correlates of behavioral inhibition using transcranial direct current stimulation. Clinical Neurology, 123: 979-984.
Jacobson, L., Koslowsky, M., Lavidor, et al. (2012). tDCS polarity effects in motor and cognitive domains: A meta-analytical review. Experimental Brain Research, 216: 1-10.
Kalu, U., Sexton, C., Loo, C, et al. (2012). Transcranial direct current stimulation in the treatment of major depression: A meta-analysis. Pyschological Medicine, 42(9): 1791-1800.
Keeser, D., Meindl, T., Bor, J., et al. (2011). Prefrontal transcranial direct current stimulation (tDCS) changes connectivity of resting-state networks during fMRI. Journal of Neuroscience, 31(43):15284-15293.
Keeser, D., Padberg, F., Reisinger, E., et al. (2011). Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: A standardized low resolution tomography (sLORETA) study. Neuroimage, 55(2): 644-657.
Khedr, E., Foly el Gamal, N., Abo el-Fetch, N., et al. (2013). Transcranial direct current stimulation and Alzheimer's disease. Clinical Neurophysiology, 124(10); e178.
Knotkova, H., Greenberg, A., Leuschner, Z., et al. (2013). Evaluation of outcomes from transcranial direct current stimulation (tDCS) for the treatment of chronic pain. Clinical Neurophysiology, 124(10):e125-e126.
Libetanz, D., Nitsche, M., Tergau, F., et al. (2002). Pharmacologic approach to the mechanisms of transcranial direct current stimulation induced after-effects of human motor cortex excitability. Brain, 125: 2228-2247.
Loo, C., Alonzo, A., Martin, D., et al. (2012). Transcranial direct current stimulation for depression: 3-week, randomized, sham-controlled trial. British Journal of Psychiatry, 200:52-59.
Maeoka, H., Matsuo, A., Hiyamizu, M., et al. (2012). Influence of transcranial direct current stimulation on pain related emotions: A study using EEG power spectrum analysis. Neuroscience Letters, 512(1): 12-16.
Marlow, N., Bonilha, H., Short, E. (2013). Efficacy of transcranial direct current stimulation and repetitive transcranial magnetic stimulation for treating fibromyalgia syndrome: A systematic review. Pain Practice, 13(2): 131-145.
Medeiros, L., Custodio de Souza, I., Vidor, L., et al. (2012). Neurobiological effects of tDCS: A review. Frontiers in Psychiatry, 3(10): 1-11.
Mehta, S., McIntyre, A., Guy, S., et al. (2015). Effect of tDCS for the management of neuropathic pain after spinal cord injury: A meta-analysis. Spinal Cord, July 2015.
Miranda, P. (2013). The electric field in the brain during tDCS. Clinical Neurophysiology, 124(10):e39.
Miranda, P., Mekonnen, A., Salvador, R., et al (2013). The electric field in the cortex during transcranial current stimulation. NeuroImage, 70: 48-58.
Mondino, M., Haesebaert, F., Poulet, E., et al. (2015). Efficacy of cathodal transcranial direct current stimulation over left orbitofrontal cortex in patient with OCD. Journal of ECT, February 2015.
Narayanaswamy, J., Jose, D., Chhabra, H., et al. (2015). Successful application of add-on transcranial direct current stimulation (tDCS) for treatment of SSRI-resistant OCD. Brain Stimulation, 8(3):655-657.
Nitsche, M., Cohen, L., Wassermann, E., et al. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1: 206-223.
O'Connell, N., Wand, B., Marston, L., et al. (2011). Non-invasive brain stimulation techniques for chronic pain. A report of a Cochrane systematic review and meta-analysis. European Journal of Physical & Rehabilitation Medicine, 47(2): 309-326.
Polania, R. (2013). tDCS and resting state fMRI. Clinical Neurophysiology, 124(10): e47.
Reis, J., Fritsch, B. (2017). Transcranial electrical brain stimulation. Neurology International Open, 1: E142-E147
Rocha, S., Melo, L., Boudoux, C., et al. (2015). Transcranial direct current stimulation in the prophylactic treatment of migraine based on interictal visual cortex excitability abnormalities: A pilot randomized controlled trial. Journal of Neurological Science, 349(1-2): 33-39.
Rossi, E., Della Torre, E., Rossi, L., et al. (2013). A multielectrode cap for transcranial direct current stimulation (tDCS). Clinical Neurophysiology, 124(10): e172.
Ruffini, G., Wendling, I., Merlet, B., et al. (2011). Transcranial current stimulation (tCS): Models and Technologies.
Saiote, C., Nemeth, D., Janacsek, K., et al. (2013). Right cathodal transcranial direct current stimulation (tDCS) over the prefrontal cortex improves implicit learning in healthy individuals. Clinical Neurophysiology, 124(10); e130.
Salehinejad, M., Rostami, R., Ghanavati, E. (2015). Transcranial direct current stimulation (tDCS) of dorsolateral prefrontal cortex (PFC) in major depression: Improving verbal working memory and reducing depression symptoms. NeuroRegulation, 2(1):37-49.
Schestatsky, P., Simis, M., Freeman,R., et al., (2013). Non-invasive brain stimulation and the autonomic nervous system. Clinical Neurophysiology, 124(9):1716-1728.
Senco, N., Huang, Y., d'Urso, G., et al. (2015). Transcranial direct current stimulation (tDCS) in OCD: Emerging clinical evidence and considerations for optimal montage of electrodes. Expert Reviews: Medical Devices, doi:10.1585/17434440.2015.1037832
Shiozawa, P., Fregni, F., Bensenor, I., et al. (2014). Transcranial direct current stimulation for major depression: An updated systematic review and meta-analysis. International Journal of Neuropsychopharmacology, 17(9): 1443-1452.
Siever, D. (2013). Transcranial DC stimulation. Neuroconnections, Spring 2013, pp.33-40.
Sparing, R., Mottaghy, F. (2008). Non-invasive brain stimulation with transcranial magnetic or direct current stimulation (TMS/TDCS): From insights into human memory to therapy of its dysfunction. Methods, 44: 329-337.
Tomlinson, S., Khusnullina, A., Bracewell, R. (2017). Non-invasive electrical brain stimulation as a treatment for depression. J. Royal College of Physicians of Edinburgh, 47: 243-245.
Turkeltaub, P., Benson, J., Hamilton, R., et al. (2012). Left lateralizing tDCS improves reading efficiency. Brain Stimulation, 5(3): 201-207.
Valle, A., Roizenblatt, S. Botte, S., et al. (2009). Efficacy of anodal tDCS for the treatment of fibromyalgia: Results of a randomized, sham-controlled longitudinal clinical trial. Journal of Pain Management, 2(3): 353-361.
Vicario, C., Nitsche, M. (2013). Transcranial direct current stimulation: A remediation tool for treatment of childhood congenital dyslexia? Frontiers in Human Neuroscience, April 2013, volume 7, article 139, pp.1-5.
Vigano, A., d'Elia, T., Sava, S., et al. (2013). Transcranial direct current stimulation (tDCS) of the visual cortex: Proof of concept study based on interictal electrophysiological abnormalities in migraine. Journal of Headache & Pain, 14: 23-32.
Wolkenstein, L., Plewnia, C. (2013). Amelioration of cognitive control in depression by transcranial direct current stimulation. Biological Psychiatry, 73: 646-651.
Yekta, M., Rostami, R., Fayyaz, E. (2015). Transcranial direct current stimulation (tDCS) of dorsolateral prefrontal cortex in patients with OCD to improve decision-making and reduce obsession symptoms. Practices in Clinical Psychology, 3(3):185-194.
Yokoi, Y., Sumiyoshi, T. (2015). Applications of transcranial direct current stimulation (tDCS) to psychiatric disorders: Trends & perspectives. Neuropsychiatric Electrophysiology, 1:10.
Annotated Bibliography of Recently Published Research Papers on the Use of tDCS to Treat Depression
Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial.
Loo, C., Alonzo, A., Martin, D., Mitchell, P., Galvez, V., Sachdev, P.
SOURCE:British Journal of Psychiatry, 2012, 200(1):52-59.
To further investigate the efficacy of tDCS in a double-blind, sham-controlled trial (registered at www.clinicaltrials.gov: NCT00763230). 64 participants with current depression received active or sham anodal tDCS to the left prefrontal cortex (2 mA, 15 sessions over 3 weeks), followed by a 3-week open-label active treatment phase. Mood and neuropsychological effects were assessed.
There was significantly greater improvement in mood after active than after sham treatment (P<0.05), although no difference in responder rates (13% in both groups).Attention and working memory improved after a single session of active but not sham tDCS (P<0.05). There was no decline in neuropsychological functioning after 3-6 weeks of active stimulation. One participant with bipolar disorder became hypomanic after active tDCS. Findings confirm earlier reports of the antidepressant efficacy and safety of tDCS. Vigilance for mood switching is advised when administering tDCS to individuals with bipolar disorder.
Enhancement of Affective Processing Induced by Bifrontal Transcranial Direct Current Stimulation in Patients With Major Depression
Andre Russowsky Brunoni, Tamires Araujo Zanao, Marie-Anne Vanderhasselt, Leandro Valiengo, Janaina Farias de Oliveira, Paulo S. Boggio, Paulo A. Lotufo, Isabela M. Benseñor, Felipe Fregni
SOURCE: Neuromodulation, May 2013.
Randomized, double-blind, sham-controlled,parallel design enrolling 24 age-, gender-matched, drug-free, depressed subjects. Anode and cathode were placed over the left and right dorsolateral prefrontal cortex. Active but not sham tDCS significantly modified the negative attentional bias. These findings add evidence that a single tDCS session transiently induces potent changes in affective processing, which might be one of the mechanisms of tDCS underlying mood changes.
The Sertraline vs Electrical Current Therapy for Treating Depression Clinical Study. Results From a Factorial, Randomized, Controlled Trial.
Andre R. Brunoni, Leandro Valiengo, Alessandra Baccaro, Tamires A. Zanão, Janaina F. de Oliveira, Alessandra Goulart, Paulo S. Boggio, Paulo A. Lotufo, Isabela M. Benseñor, Felipe Fregni
SOURCE:JAMA Psychiatry. 2013; 70(4):383-391.
The goal was to assess the combined safety and efficacy of tDCS vs a common pharmacological treatment (sertraline hydrochloride, 50 mg/d). 120 antidepressant-free patients with moderate to severe, nonpsychotic, unipolar major depressive disorder (MDD). Six-week treatment of 2-mA anodal left/cathodal right prefrontal tDCS (12 30-minute sessions: 10 consecutive sessions once daily from Monday to Friday plus 2 extra sessions every other week) and sertraline hydrochloride (50 mg/d). Use of tDCS only (but not sertraline only) was superior to placebo/sham tDCS. Common adverse effects did not differ between interventions, except for skin redness on the scalp in active tDCS (P = .03). There were 7 episodes of treatment-emergent mania or hypomania, 5 occurring in the combined treatment group. Conclusions and Relevance: In MDD, the combination of tDCS and sertraline increases the efficacy of each treatment. The efficacy and safety of tDCS and sertraline did not differ.
Trial of electrical direct-current therapy versus escitalopram for depression
Brunoni, AR., Adriano, H, Moffa, AH., et al.
SOURCE: New England J. Medicine 2017, 376: 2523-2533.
This study compares the effects of tDCS, an antidepressant medication (escitalopram), and placebo. A total of 245 of moderate to severely depressed patients were assigned to one of three treatment groups. The first group received tDCS and a placebo tablet, the second group received sham tDCS and escitalopram, and the third group received sham tDCS and a placebo tablet. The tDCS protocol consisted of the application of 30 minutes of 2.0 mA current through two 5x5 cm electrodes attached to the patients' scalps over the dorsolateral prefrontal cortices (DLPFC); with the anode over the left DLPFC and the cathode over the right DLPFC. Participants attended tDCS sessions for 15 consecutive week-days (3 weeks), followed by once-weekly sessions for the next 7 weeks. In the case of sham tDCS, participants underwent the same protocol with the exception that the current was surreptitiously turned off after 30 seconds. Participants also received either a inert placebo tablet or 10 mg of escitalopram daily for the first 3 weeks, followed by 20 mg daily for the remaining 7 weeks.
Participants in the active tDCS group were significantly more likely to report temporary mild itching, tingling, skin redness, tinnitus and nervousness than the other groups and 2 of the 91 active tDCS participants had new-onset mania/hypomania symptoms. Participants receiving escitalopram were significantly more likely to report sleepiness and constipation. Participants were far more likely to correctly guess whether they were receiving escitalopram or placebo than whether they were receiving sham or actual tDCS.
Conclusions: Level of depression as measured by the HDRS-17 questionnaire was reduced in all groups at the end of 10 weeks, with largest decrease in the escitalopram group followed by the tDCS group, and least in the placebo group. The difference between the escitalopram and tDCs groups was not statistically significant. For all intents and purposes tDCS was equivalent to escitalopram at the end of 10 weeks treatment. No post-treatment follow-up was reported.
Acute working memory improvement after tDCS in antidepressant-free patients with major depressive disorder.
Oliveira JF, Zanão TA, Valiengo L, Lotufo PA, Benseñor IM, Fregni F, Brunoni AR.
SOURCE:Neuroscience Letters. March 2013, 14:537:60-4.
28 age- and gender-matched, antidepressant-free depressed subjectsreceived a single-session of active/sham tDCS in a randomized, double-blind, parallel design. The anode was positioned over the left and the cathode over the right dorsolateral prefrontal cortex. The n-back task was used for assessing working memory and it was performed immediately before and 15min after tDCS onset. All effect sizes were large. In other words, one session of tDCS acutely enhanced WM in depressed subjects, suggesting that tDCS can improve "cold" (non affective-loaded) working memory processes in MDD.
Transcranial direct current stimulation in treatment resistant depression: a randomized double-blind, placebo-controlled study.
Palm U, Schiller C, Fintescu Z, Obermeier M, Keeser D, Reisinger E, Pogarell O, Nitsche MA, Möller HJ, Padberg F.
SOURCE:Brain Stimulation. July 2012, 5(3):242-51.
22 patients with a major depressive episodewere randomly assigned to a cross-over protocol comparing tDCS and placebo stimulation add-on to a stable antidepressant medication. Anodal tDCS, applied for 2 weeks, was not superior to placebo treatment in patients with treatment resistant depression. However, secondary outcome measures are pointing to a positive effect of tDCS on emotions. Therefore, modified and improved tDCS protocols should be carried out in controlled pilot trials to develop tDCS towards an efficacious antidepressant intervention in therapy-resistant depression.
Bifrontal tDCS prevents implicit learning acquisition in antidepressant-free patients with major depressive disorder.
Brunoni AR, Zanao TA, Ferrucci R, Priori A, Valiengo L, de Oliveira JF, Boggio PS, Lotufo PA, Benseñor IM, Fregni F.
SOURCE:Progress in Neuropsychopharmacology & Biological Psychiatry. June 2013, 43(3):146-50.
The findings for implicit (procedural) learning impairment in major depression are mixed. We investigated this issue using transcranial direct current stimulation (tDCS), a method that non-invasively increases/decreases cortical activity. 28 age- and gender-matched, antidepressant-free depressed subjects received a single-session of active/sham tDCS. We used a bifrontal setup - anode and cathode over the left and the right dorsolateral prefrontal cortex (DLPFC), respectively. The probabilistic classification-learning (PCL) task was administered before and during tDCS. The percentage of correct responses improved during sham; although not during active tDCS. Procedural or implicit learning acquisition between tasks also occurred only for sham. We discuss whether DLPFC activation decreased activity in subcortical structures due to the depressive state. The deactivation of the right DLPFC by cathodal tDCS can also account for our results. To conclude, active bifrontal tDCS prevented implicit learning in depressive patients. Further studies with different tDCS montages and in other samples are necessary.
Amelioration of cognitive control in depression by transcranial direct current stimulation.
Wolkenstein L, Plewnia C.
SOURCE:Biological Psychiatry. April 2013, 73(7):646-51.
Deficient cognitive control over emotional distraction is a central characteristic of major depressive disorder (MDD). Hypoactivation of the dorsolateral prefrontal cortex (dlPFC) has been linked with this deficit. In this study, we aimed to enhance the activity of the dlPFC in MDD patients tDCS and thus ameliorate cognitive control. In a double-blinded, balanced, randomized, sham-controlled crossover trial, we determined the effect of a single-session tDCS to the left dlPFC on the cognitive control in 22 MDD patients and 22 healthy control subjects. To assess the cognitive control, we used a delayed response working memory task with pictures of varying content (emotional vs. neutral) presented during the delay period. Emotional pictures presented during the delay period impaired accuracy and response time of patients with MDD, indicating an attentional bias for emotional stimuli. Anodal tDCS to the dlPFC was associated with an enhanced working memory performance both in patients and control subjects. Specifically in subjects with MDD, the attentional bias was completely abolished by anodal tDCS. The present study demonstrates that anodal tDCS applied to the left dlPFC improves deficient cognitive control in MDD. Based on these data, tDCS might be suitable to support the effects of behavioral training to enhance cognitive control in MDD.
Continuation transcranial direct current stimulation for the prevention of relapse in major depression.
Martin DM, Alonzo A, Ho KA, Player M, Mitchell PB, Sachdev P, Loo CK.
SOURCE:Journal of Affective Disorders. January 2013, 144(3):274-8.
Transcranial direct current stimulation (tDCS) is gaining attention as an effective new treatment for major depression. Little is known, however, of the duration of antidepressant effects following acute treatment. In this study, we describe the use of continuation tDCS treatment for up to 6 months following clinical response to an acute treatment course. 26 participants pooled from two different studies involving different tDCS protocols received continuation tDCS treatment on a weekly basis for 3 months and then once per fortnight for the final 3 months. Mood ratings were completed at 3 and 6 months. Analyses examined clinical predictors of relapse during continuation tDCS treatment. The cumulative probability of surviving without relapse was 83.7% at 3 months and 51.1% at 6 months. Medication resistance was found to be a predictor of relapse during continuation tDCS.This was an open label prospective study with no control group. Two different forms of tDCS were used. Similar to other antidepressant treatments, continuation tDCS appears to be a useful strategy to prevent relapse following clinical response. These preliminary data suggest that the majority of patients maintained antidepressant benefit with a continuation schedule of at least weekly treatment. Future controlled studies are required to confirm these findings.
Transcranial direct current stimulation (tDCS) for depression: Analysis of response using a three-factor structure of the Montgomery–Åsberg depression rating scale
Angelo Alonzo, Grace Chan, Donel Martin, Philip B. Mitchell, Colleen Loo
SOURCE:Journal of Affective Disorders, August 2013, 150(1): 91-95.
There is growing evidence that tDCS may be an effective treatment for depression. However, no study to date has profiled the antidepressant effects of tDCS using items or factors on depression symptom severity rating scales.Participants in the active tDCS treatment group showed significant improvement in dysphoria while participants in the sham treatment group did not. While both groups showed improvement in retardation symptoms, improvement was significantly greater in the active tDCS group.
Reviews
Could Transcranial Direct Current Stimulation Have Unexpected Additional Benefits in the Treatment of Depressed Patients?
The application of novel brain stimulation techniques to treat depression, and possibly other neuropsychiatric disorders, is a new and rapidly growing field. Among these techniques, transcranial direct current stimulation (tDCS) is emerging as one of the most promising approaches because of its relative ease of use, safety and neurobiological effects. One of the most promising therapeutic applications of tDCS has been in the treatment of depression. A recent meta-analysis suggested that tDCS may have robust and clinically meaningful effects in treating depression (see below). This group recently published the largest and most definitive sham-controlled trial of tDCS in depression (see above). Active stimulation was more effective than sham stimulation, and 48% of subjects who received 30 treatments of tDCS (given every weekday over a period of 6 weeks) responded to treatment. In the course of conducting this trial, it has been observed that tDCS may induce additional benefits that appeared to be independent of mood improvement. These observations are consistent with reports in the literature of cognitive enhancement and pain relief with tDCS. Anodal stimulation of the left dorsolateral prefrontal cortex (the same region stimulated for the treatment of depression) has been shown to enhance task performance across a number of 'executive' cognitive tasks, tapping higher-level cognitive functions, such as working memory, verbal fluency and planning.
Transcranial direct current stimulation in the treatment of major depression: a meta-analysis.
Kalu UG, Sexton CE, Loo CK, Ebmeier KP.
SOURCE:Psychological Medicine. September 2012, 42(9):1791-800.
Medline and Embase were searched for open-label and randomized controlled trials of tDCS in depression using the expressions ('transcranial direct current stimulation' or 'tDCS') and ('depression' or 'depressed'). Study data were extracted with a standardized data sheet. A total of 108 citations were screened and 10 studies included in the systematic review. Six randomized controlled trials were included in the meta-analysis, with a cumulative sample of 96 active and 80 sham tDCS courses. Active tDCS was found to be more effective than sham tDCS for the reduction of depression severity (Hedges' g=0.743, 95% confidence interval 0.21-1.27), although study results differed more than expected by chance (Q=15.52, df=6, p=0.017, I2=61.35). Our study was limited by the small number of studies included, which often had small sample size. Future studies should use larger, if possible representative, health service patient samples, and optimized protocols to evaluate the efficacy of tDCS in the treatment of depression further.